What is Natural Zeolite?
Natural Zeolites
are natural hydrated aluminosilicate minerals made from interlinked tetrahedra of alumina (AlO4) and silica (SiO4). Zeolite are three-dimensional crystal structure built from the elements aluminum, oxygen, and silicon, with alkali or alkaline-Earth metals (such as sodium, potassium, and magnesium) plus water molecules trapped in the gaps between them. Zeolites form with many different crystalline structures, which has large open pores in a very regular arrangement and roughly the same size as small molecules.
Zeolite Structure
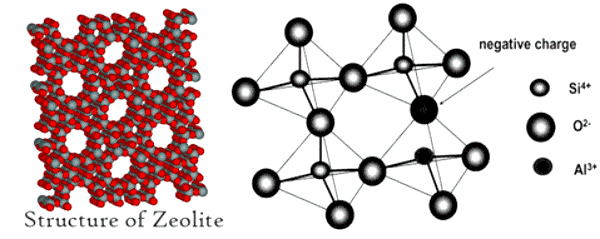 |
| Natural Zeolite Clinoptilolite Structure,Properties,Facts |
Most of the common mineral zeolites are analcime, chabazite, clinoptilolite, heulandite, natrolite, phillipsite, and stilbite. An example of the mineral formula of a zeolite is: Na 2Al 2Si 3O 10·2H2O, the formula for natrolite. These cation exchanged zeolites possess different acidity and catalyse several acid catalysis.Zeolites transform to other minerals under weathering, hydrothermal alteration or metamorphic conditions.
Synthetic zeolites hold some key advantages over their natural zeolites. The synthetic materials are manufactured in a uniform, phase-pure state. The materials of Synthetic zeolite are liquid alkali,silicate,alminum and etc. It is also possible to produce zeolite structures that do not appear in nature.
Zeolite Na-A is a well-known example. ( Antenchem is a professional manufacturer of Synthetic Zeolite in China.).Since the principal raw materials used to manufacture zeolites are silica and alumina, which are among the most abundant mineral components on earth, the potential to supply zeolites is virtually unlimited.
Zeolites are widely used as ion-exchange beds in domestic and commercial water purification, softening, and other applications. In chemistry, zeolites are used to separate molecules (only molecules of certain sizes and shapes can pass through), and as traps for molecules so they can be analyzed. Zeolites are also widely used as catalysts and sorbents. Their well-defined pore structure and adjustable acidity make them highly active in a large variety of reactions. for example,Zeolite Na-A is used as softening builder in detergent and soap. it are many type synthetic molecular sieve 3a,4a,5a,10x,13x,ZSM,MOL,NaY,and etc.
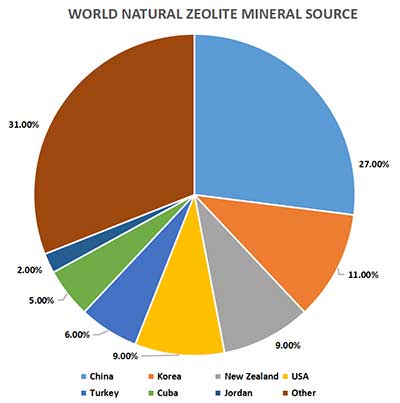 | |||||
|
World Natural Zeolite Mineral Source
Mumpton describes the zeolite as a "crystalline, hydrated aluminosilicate of alkali and alkaline earth cations having an infinite, open, threedimensional structure. It is further able to lose and gain water reversibly and to exchange extraframework cations, both without change of crystal structure. The large structural cavities and the entry channels leading into them contain water molecules, which form hydration spheres around exchangeable cations."
Zeolites
is a magnet that can hold cations, like heavy metal, ammonia, low level radioactive elements, toxins, several odours, petrochemicals, many different type of gases and a multitude of various solutions. It is a highly porous sponge with a large surface area that can absorb water up to 40% of its weight.The magic of the mineral lies at its cage structure and at its Aluminium and Silicon content which enables the mineral a high cation exchange capacity.
For more information, please visit to our partner link. If you are interested in our technology application, please contact with Mr.Lee. We would provide more support in the way of zeolite technology.
International Zeolite Association.
Xiamen Zeolitemin Biotech Co.,Ltd.
Huiying Chemical Industrial Xiamen Co., Ltd.
没有评论:
发表评论